“In-vitro Study report”
Comparison of cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company with Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga, France
Introduction
In order to perform the In-vitro study according to the general Chapter of USP42/NF37 <1088>, <1090> Monograph method, and the Comparison of cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company with Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga, France with the following tests, their full description is attached
Comparison of L-Cystine release from 12 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 12 Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga, France, according to the internal method and general method <711> in USP 42 and calculation of similarity factor and The difference factor
Assay of L-Cystine, L-arginine, vitamin B6 and zinc in cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga company in France according to In-House method
Evaluation of uniformity of drug content (Uniformity of dosage form) according to the general method of weight variation in USP 42 / NF 37, <2091> in cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga company France
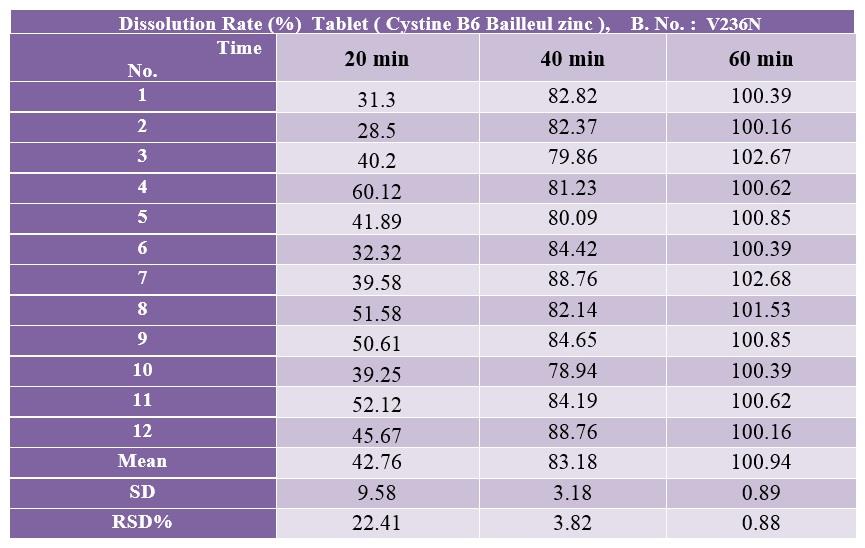
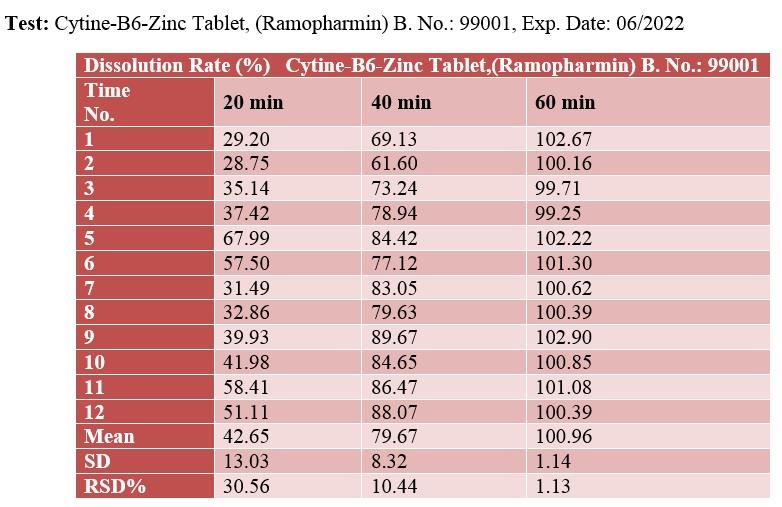
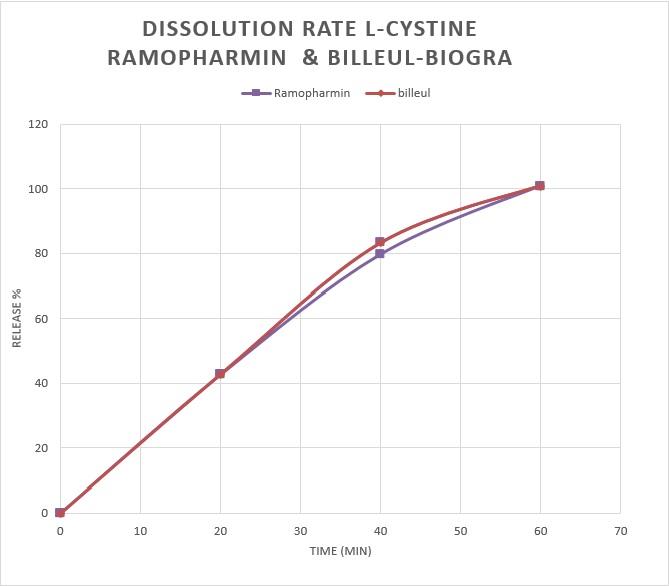
Comparison of L-Cystine release from 12 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 12 Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga, France
Method of preparing the standard solution
A solution with a concentration of 0.555 mg / ml L-Cystine was prepared from the L-Cystine reference standard powder in the dissolution medium
Method of preparing the sample solution
The release of L-Cystine from 12 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 12 Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga, France was evaluated and compared according to the following conditions
Apparatus II (Paddle)
Speed: 100 rpm
Medium: 900 ml HCl 0.01 N
Time: 60 min
At a time of 20, 40 and 60 minutes, sampled 10 ml of the solution in each of the Dissolution tester vessels and Filtrated with a nylon filter, pore size 0.45 microns, and the concentration of sample and standard solutions was calculated according to the following method
Chromatographic Condition
Column: C8 – 4.6mm × 250mm, 5µm
Wavelength: 215nm
Flow: 1ml/min
Injection volume: 100 µL
Method
Inject the standard solution 5 times, so that the relative standard deviation should not be more than 2%, Also, the performance of the column should not be less than 1500 theoretical plates
Calculation method
Based on the area below the peaks obtained from Standard and Test Solutions, we calculate the percentage of L-Cystine dissolution using the following formula
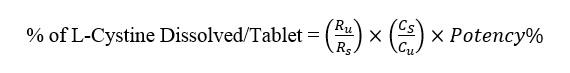
In the above equation
Ru: peak response resulting from the injection of the sample solution
Rs: Average peak response resulting from standard solution injection
Cs: Standard solution concentration in mg / ml
Cu: Sample solution concentration in mg / ml
P%: Potency of Reference standard of L-Cystine
Result
The amount of L-Cystine released from each of the sample and reference tablets after 60 minutes should not be less than 75% (Q = 75%) of the amount mentioned on the product label
Similarity factor
To evaluate the similarity and proximity of the formulation of sample and reference tablets in this comparison, the similarity factor is calculated according to the following formula
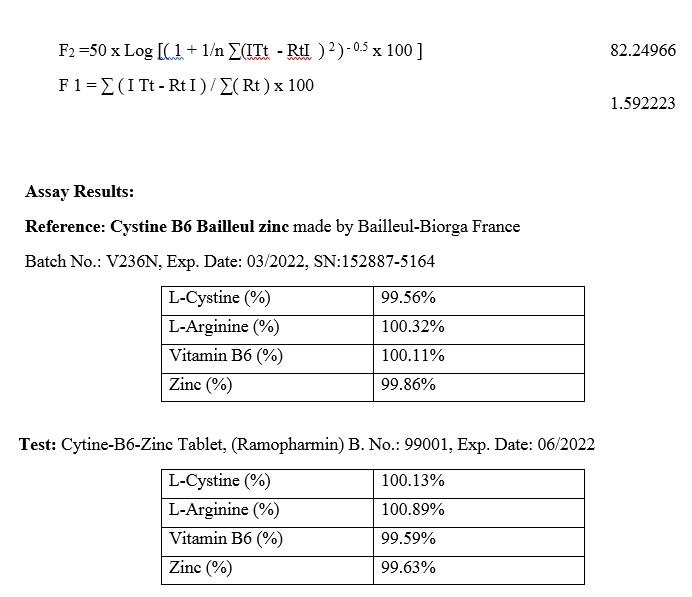
F2=50*log {[1+ (1/n) Σ (Rt-Tt) 2]-0.5*100}
N=Number of time points
Rt&Tt: The cumulative percentage dissolved at each of selected n time points of the reference & test respectively
Results
The value obtained should be in the range of (50-100) percent
Difference factor
To investigate the difference between the percentage of dissolution and release of sample and reference tablets in this comparison, the Difference factor is calculated according to the following formula
F1=(ΣIRt-TtI/ΣRt)*100
Results
The value obtained should be in the range of (0-15) percent
Assay comparison
Assay of L-Cystine, in cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga company in France
This test method is performed according to the method mentioned in USP 42 / NF37 monograph
Method of preparing the standard solution
Weigh 20 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 20 Cystine B6 Bailleul zinc supplements made by Bailleul-Biorga France and then powder
Using some of these powder, prepare 1 mg / ml Solution of L-Cystine and make up to volume with 0.1 N hydrochloric acid solution
Diluent: 0.1 N hydrochloric acid solution
Solution A: Solution of 1g of 1-octane Sulfonic acid salt in 2000ml of phosphate buffer pH=3.5
Mobile Phase: Acetonitrile: Solution A (10:190)
Filter: Nylon 0.45µm
Chromatographic condition
Column: C8 – 4.6mm × 250mm, 5µm
Wavelength: 215nm
Flow: 1ml/min
Injection volume: 100 µL
Method
Inject the standard solution 5 times, so that the relative standard deviation should not be more than 2%, Also, the performance of the column should not be less than 1500 theoretical plates
Calculation method
Using HPLC according to in-house method, based on the area below the peaks obtained from Standard and Test Solutions, calculate the Assay of L-Cystine( in mg in the portion of tablet taken
The assay of L-Cystine in the amount of powder taken from the tablet is equivalent to 500 mg of L-Cystine and can be measured by the following method
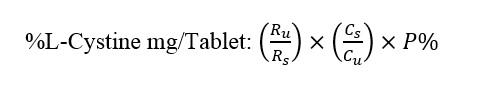
In the above equation
Ru: peak response resulting from the injection of the sample solution
Rs: Average peak response resulting from standard solution injection
Cs: Standard solution concentration in mg / ml
Cu: Sample solution concentration in mg / ml
P%: Potency of Reference standard of L-Cystine
Results
The assay of L-Cystine calculated in the sample and reference tablets should be in the range of (90-110) percent of the amount mentioned on the product label
Assay of L-Arginine, in cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga company in France
This test method is performed according to the method mentioned in USP 42 / NF37 monograph
Method of preparing the standard solution
A solution with a concentration of 0.15 mg / ml L-Arginine was prepared from the L-Arginine reference standard powder in the Phosphate Buffer Solution
Method of preparing the sample solution
Weigh 20 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 20 Cystine B6 Bailleul zinc supplements made by Bailleul-Biorga France and then powder
Using some of these powder, prepare 0.15 mg / ml Solution of L-Arginine and make up to volume with Phosphate buffer solution
Diluent: phosphate buffer pH=3.5 solution
Solution A: Solution of 1g of 1-octane Sulfonic acid salt in 2000ml of phosphate buffer pH=3.5
Mobile Phase: Acetonitrile: Solution A (10:190)
Filter: Nylon 0.45µm
Chromatographic condition
Column: C8 – 4.6mm × 250mm, 5µm
Wavelength: 215nm
Flow: 1ml/min
Injection volume: 100 µL
Method
Inject the standard solution 5 times, so that the relative standard deviation should not be more than 2%, Also, the performance of the column should not be less than 1500 theoretical plates
Calculation method
Using HPLC according to in-house method, based on the area below the peaks obtained from Standard and Test Solutions, calculate the Assay of L-Arginine ( in mg in the portion of tablet taken
The assay of L-Arginine in the amount of powder taken from the tablet is equivalent to 1.5 mg of L-Arginine and can be measured by the following method
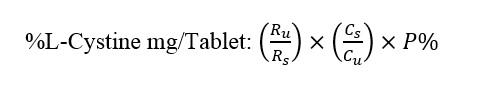
In the above equation
Ru: peak response resulting from the injection of the sample solution
Rs: Average peak response resulting from standard solution injection
Cs: Standard solution concentration in mg / ml
Cu: Sample solution concentration in mg / ml
P%: Potency of Reference standard of L-Arginine
Results
The assay of L-Arginine calculated in the sample and reference tablets should be in the range of (90-110) percent of the amount mentioned on the product label
Assay of Vitamin B6, in cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga company in France
This test method is performed according to the method mentioned in USP 42 / NF37 monograph
Method of preparing the standard solution
A solution with a concentration of 0.007 mg / ml Pyridoxine HCl was prepared from the Pyridoxine HCl reference standard powder in the Hydrochloric acid 0.1N Solution
Method of preparing the sample solution
Weigh 20 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 20 Cystine B6 Bailleul zinc supplements made by Bailleul-Biorga France and then powder
Using some of these powder, prepare 0.007 mg / ml Solution of Pyridoxine HCl and make up to volume with 0.1 N hydrochloric acid solution
Diluent: 0.1 N hydrochloric acid solution
Mobile Phase: 270 ml of methanol, 10 ml of glacial acetic acid, 730 ml of pure water with 1.4 g of sodium 1-hexane sulfonate
Filter: Nylon 0.45µm
Chromatographic condition
Column: C18 – 4.6mm × 150mm, 5µm
Wavelength: 280 nm
Flow: 1ml/min
Injection volume: 100 µL
Method
Inject the standard solution 5 times, so that the relative standard deviation should not be more than 2%, Also, the performance of the column should not be less than 1500 theoretical plates
Calculation method
Using HPLC according to in-house method, based on the area below the peaks obtained from Standard and Test Solutions, calculate the Assay of Pyridoxine HCl in mg in the portion of tablet taken
The assay of Pyridoxine HCl in the amount of powder taken from the tablet is equivalent to 0.35 mg of Pyridoxine HCl and can be measured by the following method
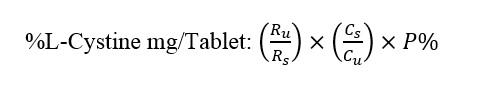
In the above equation
Ru: peak response resulting from the injection of the sample solution
Rs: Average peak response resulting from standard solution injection
Cs: Standard solution concentration in mg / ml
Cu: Sample solution concentration in mg / ml
P%: Potency of Reference standard of Pyridoxine HC
Results
The assay of Pyridoxine HCl calculated in the sample and reference tablets should be in the range of (90-110) percent of the amount mentioned on the product label
Assay of Zinc, in cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga company in France
This test method is performed according to the method mentioned in USP 42 / NF37 monograph
Method of preparing the sample solution
Weigh 20 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 20 Cystine B6 Bailleul zinc supplements made by Bailleul-Biorga France and then powder
weigh some of these powder, equivalent to 2.5mg elemental zinc and pour into an 500ml Erlenmeyer
Method: Titration
Titrant Solution: 0.001M EDTA Solution
End Point: color change observation
Blank: the same solution as Sample Solution without the API
Procedure
Add 15 ml of acetic acid to your sample and add 200 ml of water and 50 mg of Xylenol Orange Triturate indicator. Neutralize the solution with 2 mg of methylamine. Titrate the sample and control solution with EDTA Solution
Calculation method
The assay of elemental zinc in the amount of powder taken from the tablet is equivalent to 2.5 mg of elemental zinc and can be measured by the following method
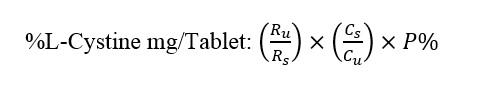
In the above equation
VS Titrant consumption volume for sample solution
VB Titrant consumption volume for control solution
M: Molarity of titrant mmol / ml
F: Equivalent factor 39/65 mg / mmol
W: Zinc uptake in the sample in milligrams
Results
The assay of elemental zinc calculated in the sample and reference tablets should be in the range of (90-110) percent of the amount mentioned on the product label
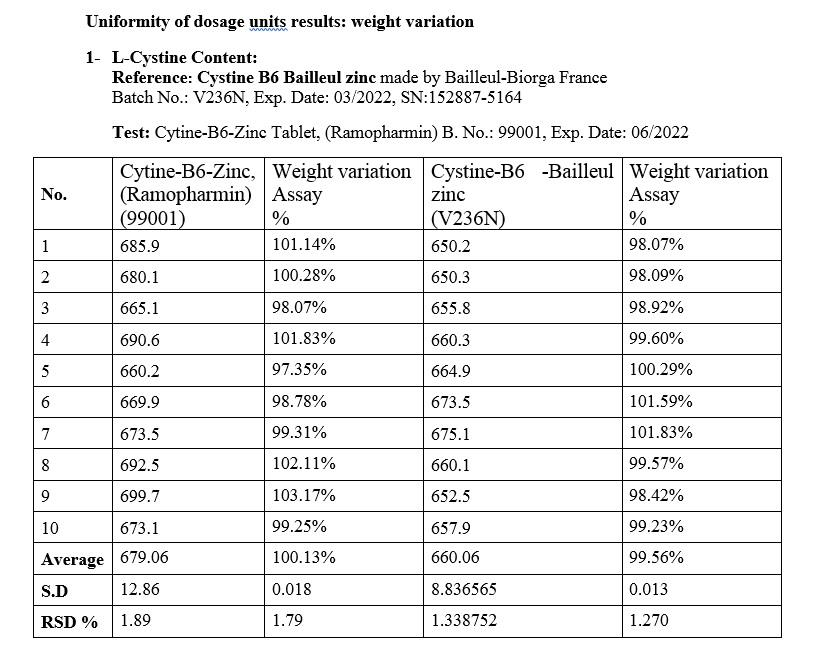
Investigating the uniformity of dosage form in cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and Cystine B6 Bailleul zinc tablets made by Bailleul-Biorga company in France
This test method is performed according to the general method mentioned in USP 42 / NF37, <905> by weight variation method
Weight Variation: Weigh 10 cystine-B6-zinc supplement tablets made by Ramopharmin Pharmaceutical Company and 10 Cystine B6 Bailleul zinc supplements made by Bailleul-Biorga France separately and calculate the amount of L-Cystine( in mg using the assay method. Relative standard deviation should not be more than 6%
Results
Cystine-B6-Zinc supplement tablets contain 500 (425-575) mg of L-Cystine, equivalent to 85 to 115% of the amount indicated on the label
In-vitro Result
Dissolution report
Reference: Cystine B6 Bailleul zinc made by Bailleul-Biorga France
Batch No.: V236N, Exp. Date: 03/2022, SN:152887-5164